Global Prefilled Syringes Market was Valued at USD 18.42 Billion in 2022 and is Expected to Reach USD 54.06 Billion In 2030, with a CAGR of 12.48% During the Forecast Period 2023-2030.
By Infinium Global Research Jan, 2024
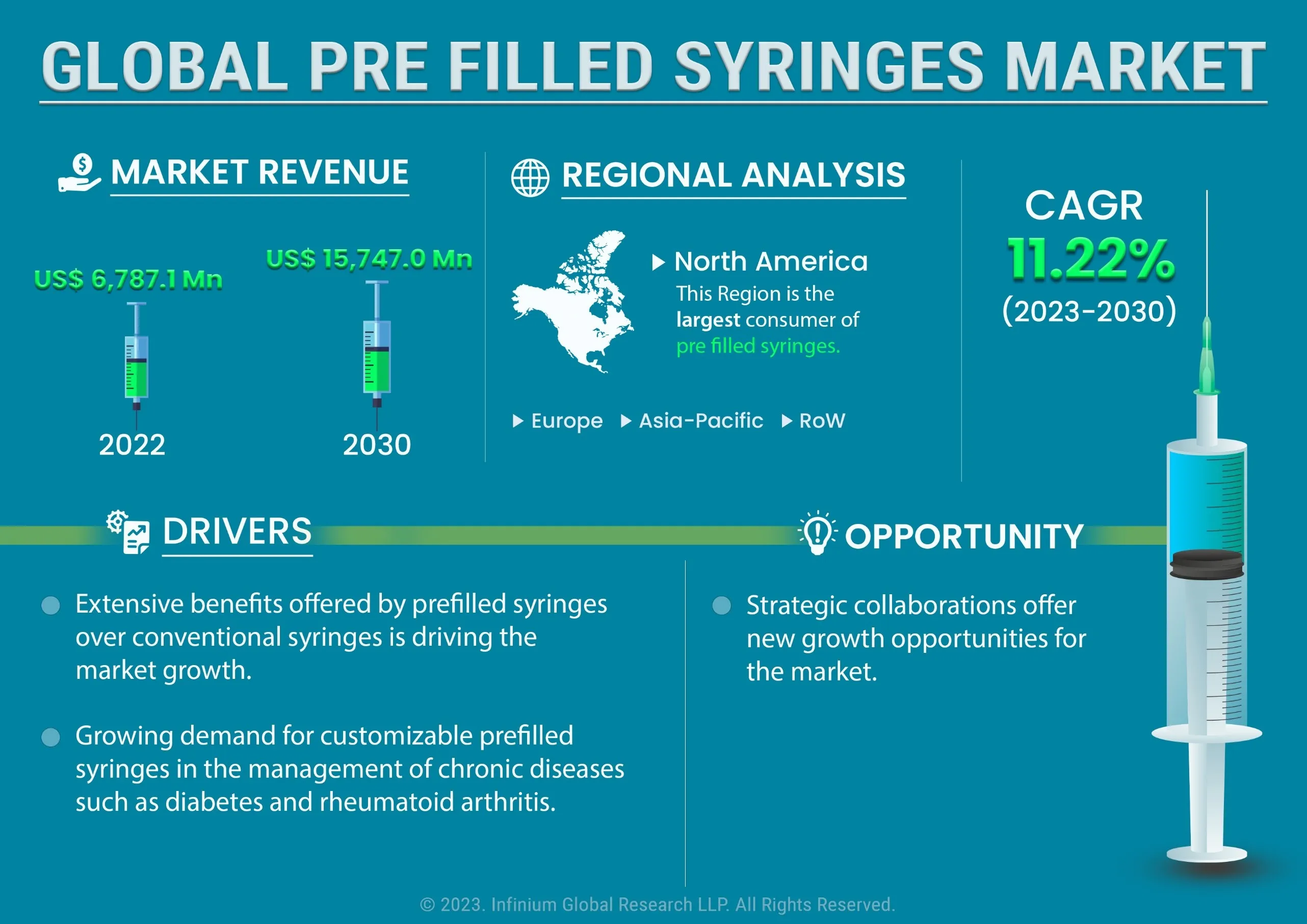
The global prefilled syringes market was valued at USD 6,787.1 million & 7,887 million Units in 2022 and is expected to reach USD 15,747.0 million & 15,816 million Units by 2030 and grow at a CAGR of 11.22% & 9.21% over the forecast period. Extensive benefits offered by prefilled syringes over conventional syringes is driving the market growth. Compared to traditional vial and syringe combinations, prefilled syringes offer multiple benefits. Healthcare workers face a substantial risk of needlestick injuries from syringes, leading to the transmission of bloodborne diseases like HIV, hepatitis B, and hepatitis C. The World Health Organization (WHO) reports that about 3 million healthcare workers experience needlestick injuries annually, while the Centers for Disease Control and Prevention (CDC) estimates approximately 385,000 needlestick injuries per year in the United States. Further, Growing demand for customizable prefilled syringes in the management of chronic diseases such as diabetes and rheumatoid arthritis. According to IDF Diabetes Atlas 537 million adults are living with diabetes and the number of diabetic patients is expected to grow to 643 million by 2030 and 783 million by 2045. The healthcare expenditure related to diabetes was at least USD 966 billion in 2021. Diabetic patients need to be continuously administered by insulin. Insulin wastage is a major concern now a days. Furthermore, customized prefilled syringes are being actively used in pain management, in the treatment of Multiple sclerosis, oncology treatments and many others. However, Stringent Regulatory Landscape and Intellectual Property Rights may restrain the growth of the market. Regulations governing medical devices are becoming more rigorous, especially in developed countries such as the US and Europe, with the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) imposing strict approval requirements for devices, including prefilled syringes. These requirements could result in increased approval costs and longer timelines, potentially creating barriers to entry for smaller companies seeking to compete in the market. Additionally, Strategic collaborations offer new growth opportunities for the market. By bringing together complementary technologies, expertise, and resources, strategic collaborations present new opportunities for the prefilled syringes market to create innovative solutions. Furthermore, strategic collaborations can provide access to emerging markets, which can drive market penetration and growth for prefilled syringes.
