Biocompatibility Testing Services for Medical Devices Market (Service - Biocompatibility Test, Chemistry Test, Microbiology & Sterility Testing, and Package Validation; Phase - Preclinical, and Clinical): Global Industry Analysis, Trends, Size, Share and Forecasts to 2030
A recent report published by Infinium Global Research on biocompatibility testing services for medical devices market provides in-depth analysis of segments and sub-segments in the global as well as regional biocompatibility testing services for medical devices market. The study also highlights the impact of drivers, restraints, and macro indicators on the global and regional biocompatibility testing services for medical devices market over the short term as well as long term. The report is a comprehensive presentation of trends, forecast and dollar values of global biocompatibility testing services for medical devices market.
Market Insight:
Biocompatibility testing services for medical devices are essential to ensure product safety and regulatory compliance. These services assess the compatibility of medical devices with the human body, minimizing the risk of adverse reactions or complications. Biocompatibility testing evaluates various aspects, such as cytotoxicity, sensitization, irritation, and systemic toxicity. It also examines the device's materials and manufacturing processes. By outsourcing these services to specialized providers, medical device companies can save time and resources while benefiting from expert knowledge and state-of-the-art testing facilities. Ensuring biocompatibility not only safeguards patient health but also enhances the reputation and market acceptance of medical devices. In today's stringent regulatory environment, partnering with trusted biocompatibility testing services is a prudent business strategy to expedite product development and market entry.
Regulatory compliance indeed serves as a substantial driver for the growth of the market. Regulatory bodies, such as the FDA in the United States and the EU's MDR (Medical Device Regulation), mandate comprehensive biocompatibility testing for medical devices. Compliance is essential to obtain market approvals and ensure patient safety. Evolving regulations, with an increased focus on risk management, demand more extensive and rigorous testing, propelling the demand for specialized testing services. Additionally, increasing technological advancements are boosting the growth of the market. Rapid advancements in medical device technology and materials necessitate ongoing biocompatibility assessment. Biocompatibility testing services providers continuously adapt and develop new testing methodologies to address these emerging technologies, making their expertise indispensable to device manufacturers seeking to bring cutting-edge products to market while mitigating potential risks. However, the cost and time associated with comprehensive biocompatibility testing may act as a potential restraint to the growth of the market. Furthermore, the increasing demand for biocompatibility testing services creates a lucrative opportunity for the growth of biocompatibility testing services for the medical devices market.
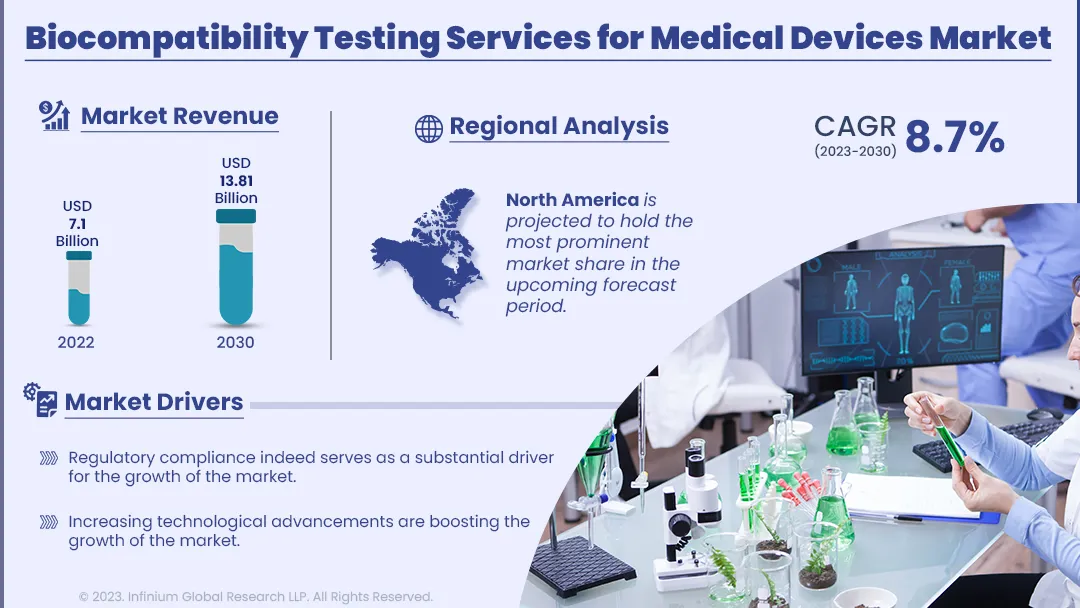
The biocompatibility testing services for medical devices is segmented into North America, Asia Pacific, Europe, and ROW. Among this region, North America is projected to hold the most prominent market share in the upcoming forecast period. This supremacy is attributed to the stringent regulatory environment set by the U.S. Food and Drug Administration (FDA), making comprehensive biocompatibility testing a necessity for market approval. The presence of a robust medical device industry in the United States, including established manufacturers and innovative startups, further fuels the demand for these services. Moreover, the Asia-Pacific region is the fastest-growing market for biocompatibility testing services for medical devices. This rapid growth can be attributed to several key factors. Firstly, Asia-Pacific countries are experiencing a surge in medical device manufacturing, driven by cost-effective production capabilities and a rising demand for healthcare solutions. Additionally, regulatory bodies in the region, such as China's National Medical Products Administration (NMPA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA), are increasingly aligning their standards with international best practices, necessitating more extensive biocompatibility testing services.
Report Scope of the Biocompatibility Testing Services for Medical Devices Market:
| Report Coverage | Details |
|---|---|
| Market Size in 2022 | USD 7.1 Billion |
| Market Size by 2030 | USD 13.81 Billion |
| Growth Rate from 2023 to 2030 | CAGR of 8.7% |
| Largest Market | North America |
| No. of Pages | 180 |
| Market Drivers |
|
| Market Segmentation | By Service, and By Phase |
| Regional Scope | North America, Europe, Asia Pacific, and RoW |
Segment Covered
The report on global biocompatibility testing services for medical devices market covers segments such as service, and phase. On the basis of service, the sub-markets include biocompatibility test, chemistry test, microbiology & sterility testing, and package validation. On the basis of phase, the sub-markets include preclinical, and clinical.
Companies Profiled:
The report provides profiles of the companies in the market such as North American Science Associates, LLC, Wickham Laboratories Ltd, GLR Laboratories Pvt Ltd, Nelson Laboratories LLC, ACCUPREC RESEARCH LABS PVT. LTD, Labcorp, Pacific BioLabs, Inc., Biocomp Laboratories, Morulaa HealthTech Pvt. Ltd., and Geneva Laboratories Inc.
Report Highlights:
The report provides deep insights into demand forecasts, market trends, and micro and macro indicators. In addition, this report provides insights into the factors that are driving and restraining the growth in this market. Moreover, The IGR-Growth Matrix analysis given in the report brings an insight into the investment areas that existing or new market players can consider. The report provides insights into the market using analytical tools such as Porter's five forces analysis and DRO analysis of the biocompatibility testing services for medical devices market. Moreover, the study highlights current market trends and provides forecasts from 2023-2030. We also have highlighted future trends in the market that will affect the demand during the forecast period. Moreover, the competitive analysis given in each regional market brings an insight into the market share of the leading players.
Please Choose One of them.